Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Histopathological, Scanning and Transmission Electron Microscopic Study of Fluoride Induced Intestinal Lesions in Wistar Rats
*Corresponding author: Shashi A, Department of Zoology and Environmental Sciences, Punjabi University, Patiala-147002, India.
Received: August 05, 2024; Published: August 09, 2024
DOI: 10.34297/AJBSR.2024.23.003102
Abstract
The present study evaluated the toxic effects of fluoride-induced ultrastructural changes in the small intestine of albino rats. Wistar albino rats were divided into two groups. The control group received 1 ml of deionized water daily for 40 days via oral gavage, while the experimental group was treated with 600 mg/kg b.w./day of sodium fluoride for the same period. At the end of the experimentation period, the rats were sacrificed, and samples from the small intestine (duodenum, jejunum, and ileum) were collected for histopathological, scanning and transmission electron microscopy. The results showed that rats treated with 600mg/kg b.w./day of sodium fluoride exhibited various gastrointestinal abnormalities, including scattered villi, disrupted villus architecture, swollen cells, widened cryptic mouths, corrugations, irregular nuclei, vacuolations, and loss of microvilli. Additionally, there was widening of spaces and disruption of mitochondria, and the endoplasmic reticulum lost its characteristic appearance.
Keywords: Albino rats, Histopathology, Scanning electron microscopy, Small intestine, Sodium fluoride, Transmission electron microscopy, Wistar rats
Introduction
Fluorosis is a form of chronic fluoride intoxication resulting from ingestion of excessive quantities of fluoride through drinking water [1]. Excessive ingestion of fluoride not only causes skeletal and dental fluorosis [2,3] but also leads to gastrointestinal disturbances. As fluoride can penetrate cell membranes, long-term exposure may result in significant gastrointestinal problems, neurological disorders [4] and cardiomyopathy [5]. Gastrointestinal symptoms have been described to be crucial indicators of fluoride toxicity and complaints such as nausea, vomiting, diarrhoea and abdominal pain have been reported [6].
The gastrointestinal system is thought to be the main route of fluoride exposure because of its significant involvement in fluoride absorption. Fluoride is absorbed from the intestine through a passive diffusion process [7]. Acute and chronic studies on experimental animals and humans have demonstrated that consuming fluoride causes gastrointestinal damage [8,9]. The small intestine plays a crucial role in fluoride absorption, have significantly higher fluoride concentrations that the other tissues. The intestine plays a key role in several functions which include digestion and absorption of carbohydrates, fats and proteins and absorption of vitamins and minerals. Exposure to environmental toxins and physical stress has been associated with a rise in gastrointestinal issues, making it a significant societal concern. This study elucidated the microscopic changes in the small intestine caused by sodium fluoride toxicity, using techniques like histopathology, scanning electron microscopy, and transmission electron microscopy.
Materials and Methods
Wistar albino rats weighing 150-200 g were housed in polypropylene cages with stainless steel grill tops and fed with standard commercial rat pellet diet (Hindustan Lever Limited, Mumbai, India) and water was given ad libitum. After acclimatization for two weeks, the animals were divided into two groups and each group consisted of six rats. Group 1 served as control receiving deionized water for 40 days via oral gavage. Group II was administered 600 mg/kg b.w./day of sodium fluoride in drinking water for the same period. The animals were sacrificed at the end of experimentation period and the organs from small intestine (duodenum, jejunum and ileum) were taken out and processed for histopathological, scanning and transmission electron microscopy. Small parts of the duodenum, jejunum and ileum were excised immediately and fixed in alcoholic Bouin’s fluid for light microscopic study. Specimens were dehydrated, cleared and embedded in Paraffin wax. Sections of 7µm in thickness were stained with haematoxylin and eosin [10]. The stained sections were studied under the research microscope (Leica microsystem) and subsequently photomicrography. The cytoplasm appeared reddish-pink, and the nuclei acquired a blue colour.
For scanning electron microscopic examination, various parts of small intestine (viz; duodenum, jejunum and ileum) were fixed in phosphate buffered 2.5% glutaraldehyde and 2% paraformaldehyde [11]. and post-fixed in phosphate buffered 1 % osmium tetroxide for 2 hours. The specimens were dehydrated in graded series of acetone and dried at a critical point using liquid CO2. The dried specimens were mounted on aluminium stubs and then coated with gold and observed under (JEOL JSM-6510) scanning electron microscope at an operating voltage 15kV. Images were digitally acquired using a CCD camera attached to the microscope at All India Institute of Medical Sciences, New Delhi, India. Other pieces of small intestine were fixed in 2.5% phosphate buffered glutaraldehyde at 4°C for 24 hours and washed 3-4 times in 0.1M phosphate buffer, post-fixed in 1% osmium tetroxide, and then dehydrated. The semithin sections were stained with toludene blue and the ultrathin sections stained with uranyl acetate and lead citrate and examined and on transmission electron microscope (Tecnai 2 Fei Company, The Netherlands).
Results
Light Microscopic Examination
The duodenum of control rat showed histological features such as presence of different layers such as mucosa, submucosa, muscularis and serosa. The lining of epithelium of microvilli was composed of many cell types such as goblet cells, Paneth cells and absorptive columnar epithelial cells (Figure 1). Brünner’s glands were seen in the submucosal region of the duodenum which secrete alkaline fluid containing mucin (Figure 2). The rats treated with 600mg/kg b.w./day for 40 days revealed thickened and scattered villi (Figure 3). Hypertrophy of goblet cells and damaged crypts were also observed. (Figure 4). The necrotic Brünner’s gland showed inflammation and dilation of spaces (Figure 5).
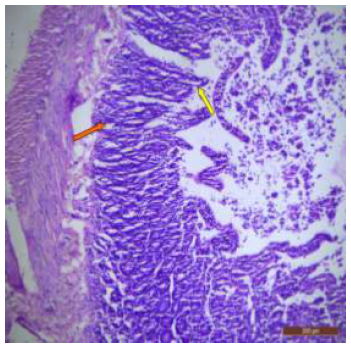
Figure 1: T.S. of duodenum of control rat showing crypts (↑), closely packed microvilli (↑), well developed submucosa and muscularis externa. H&E × 100.
There was disruption in the villus architecture with crypt loss. The submucosa, muscularis externa and outer serosa were undifferentiated and were not clearly seen (Figure 6). There was marked villous atrophy, characterized by the shrinking and flattening of the intestinal villi. Additionally, mucosal erosion was also evident, indicating the wearing away of the protective mucosal layer (Figure 7). The duodenal tissues showed widespread disruption, with visible signs of structural disorganization and inflammation (Figure 8). The jejunum of control rat showed distinctive layers, i.e; mucosa, submucosa, muscularis and serosa (Figure 9). The jejunal mucosa was formed of epithelium, lamina propria and muscaris mucosa. The mucosa appeared as finger like projections with a core of connective tissue covered with enterocytes. Goblet cells were also seen in between the cells which are specialized type of epithelial cells, secrete mucus to neutralize the acids produced by stomach (Figures 10,11).

Figure 2: T.S. of duodenum of control rat showing Brunner’s gland with darkly stained nuclei (↑). H&E × 400.>/p>

Figure 3: T.S. of duodenum of rat treated with 600 mg NaF/kg bw/day for 40 days showing thickened and scattered villi. H&E × 100.
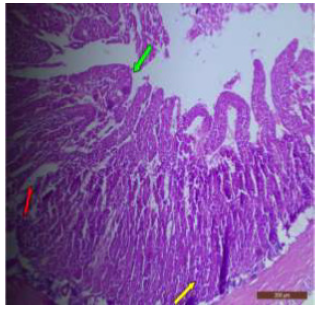
Figure 4: T. S. of duodenum of rat treated with 600 mg NaF/kg bw/ day for 40 days showing bulbous and swollen villus tip (↑), damaged crypts (↑) and hypertrophy of goblet cells (↑). H&E × 100.
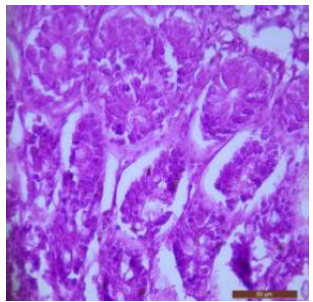
Figure 5: T.S. of duodenum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing necrosis of Brunner’s gland. H&E × 400.
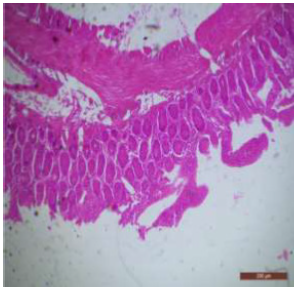
Figure 6: T.S. of duodenum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing absence of villi (↑), distorted layers (↑) and loss of crypts (↑). H&E × 100.

Figure 7: T.S. of duodenum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing villous atrophy (↑) and mucosal erosion (↑). H&E × 40.
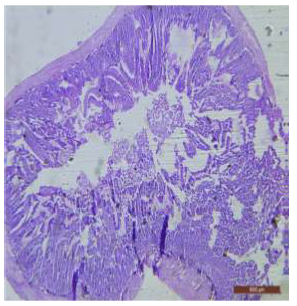
Figure 8: T.S. of duodenum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing widespread disruption and structural disorganization. H&E × 40.

Figure 9: T.S. of jejunum of control rat showing mucosa (↑), submucosa (↑), muscularis mucosa (↑) and serosa (↑). H&E × 100.

Figure 10: T.S. of jejunum of control rat showing long finger like villi (↑) with a core of connective tissue covered with enterocytes and goblet cells (↑). H&E × 100.
In fluoridated rats damaged and broken villi, focal intervillous haemorrhages and ulceration was present (Figure 12). There was shortening and flattening of some of the villi (Figure 13), distorted crypts, large swollen goblet cells lined by columnar epithelium with wide spaces were present (Figure 14). Distortion of different layers of mucosa with the loss of villous architecture and shedding of surface epithelium was seen in the jejunum of fluoridated rats (Figure 15). The villus architecture was notably disturbed, with a marked loss of crypts. Furthermore, there was a noticeable decrease in the number of goblet cells (Figure 16). The villi appeared scattered and fragmented, indicating a disruption in their typical architecture. Reduction in size of intestinal glands highlighted the detrimental impact of fluoride exposure on the intestinal morphology of rats (Figure 17).
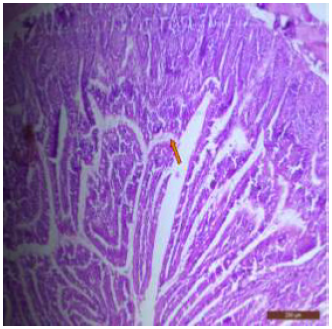
Figure 12: T.S. of jejunum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing ulceration, focal intravillous hemorrhages and broken villi (↑). H&E × 100.
The ileal mucosa of control rat was built up of numerous folds forming the villi, through which connective tissue of the lamina propria containing tubular glands i.e., crypts of Leiberkühn were present (Figure 18). The epithelial lining of the villi was composed of Paneth cells and intestinal glands (Figure 19). In fluorotic rats, there were abnormal shaped, damaged and broken villi with bulbous tips. Lymphatic dilation and haemorrhaged in lamina propria were also seen (Figure 20). In the ileal mucosa, prominent inflammation, distortion of crypts with shredded cells inside the crypts were visible (Figure 21). Swollen and necrotic crypts were present (Figure 22). The ileal tissue showed disruptions in the normal mucosal architecture with signs of villous atrophy and cell infiltration (Figure 23). Lymphatic infiltration was prominent and numerous enlarged lymph nodules appeared in lamina propria of villi. There were ill-defined cell boundaries, vacuolated cytoplasm and pyknotic nuclei (Figure 24).

Figure 13: T.S. of jejunum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing distortion, shortening and flattening of villi (↑). H&E × 100.

Figure 14: T.S. of jejunum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing swollen goblet cells and few nuclei (↑). H&E× 400.
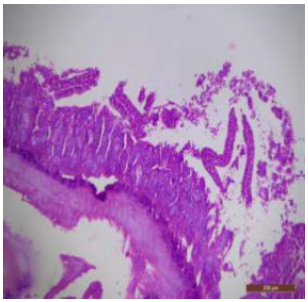
Figure 15: T.S. of jejunum of rat treated with 600 mg NaF/ kg b.w./day for 40 days showing atrophy of villi and distortion of different layers and destruction of surface epithelium (↑). H&E × 100.
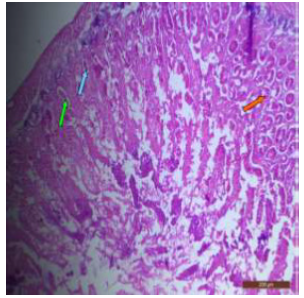
Figure 16: T.S. of jejunum of rat treated with 600 mg NaF/ kg b.w./day for 40 days showing disruption of villi (↑), reduction in number of goblet cells (↑) and crypt loss (↑). H&E × 100.
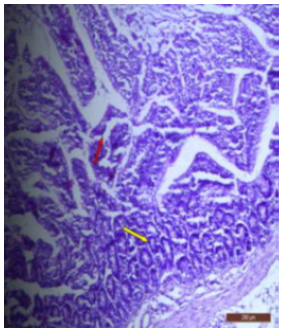
Figure 17: T.S. of ileum of control rat showing scattered and broken villi (↑) and decrease in size of intestinal glands (↑). H&E × 100.
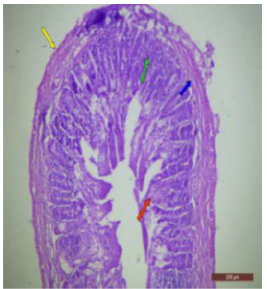
Figure 18: T.S. of ileum of control rat showing mucosa (↑), submucosa (↑), muscularis externa (↑) and serosa (↑). H&E × 100.
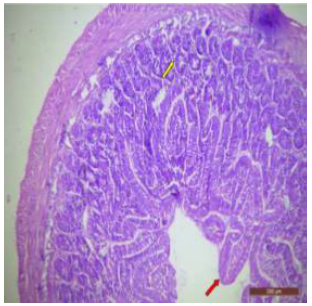
Figure 19: T.S. of ileum of control rat showing normal villi epithelial lining, crypts of Leiberkuhn (↑) and submucosa. Below the villi, the intestinal glands (↑) are present. H&E × 100.
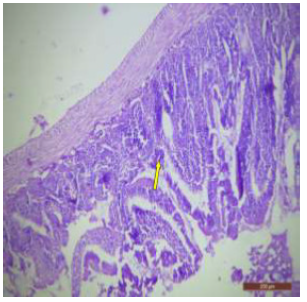
Figure 20: T.S. of ileum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing broken villous and necrotic crypts (↑).H&E × 100.
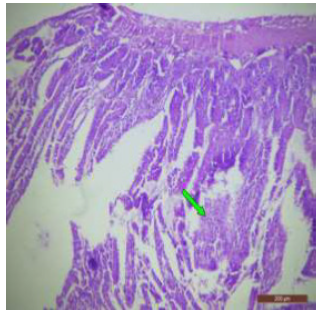
Figure 21: T.S. of ileum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing inflammation (↑) in the mucosa. H&E × 100.
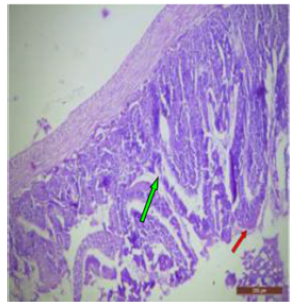
Figure 22: T.S. of ileum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing swollen (↑), broken villus and necrotic tips (↑). H&E × 100.
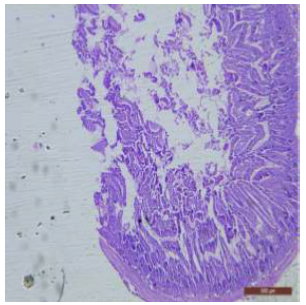
Figure 23: T.S. of ileum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing disrupted mucosal structure. H&E × 40.
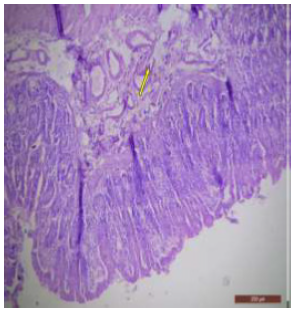
Figure 24: T.S. of ileum of rat treated with 600 mg NaF/kg b.w./day for 40 days showing abnormalities in the lymph nodes (↑). H&E × 100.
Scanning Electron Microscopic Changes
The scanning electron microscopic examination of duodenum in control rat revealed goblet cells distended with mucus in between the enterocytes (Figure 25). The epithelial cells with long and short microvilli were present (Figure 26). The rats treated with 600 mg NaF/kg b.w./day for 40 days, revealed apparent erosions and fissures at the tips of duodenal villi surrounded by enterocytes (Figure 27). There were extravasation of red blood cells observed at specific sites of villous surface (Figure 28). On the surfaces of the crypt orifices, villi appeared to be lacking. The fluorotic rats have wider crypt mouths and corrugations in their duodenum. (Figure 29,30). The villus had deep irregular corrugations. The goblet cell orifices were also visible in the duodenum of fluoridated rats (Figure 31). Significant damage characterized by erosions on the mucosal surface and leaf-like villi showed signs of autolysis. Presence of bleb was observed in the duodenal mucosa of fluoridated rats indicating injury (Figure 32).

Figure 25: Scanning electron micrograph of duodenum of control rat showing goblet cells (↑) distended with mucus in between enterocytes (↑). X 1980.
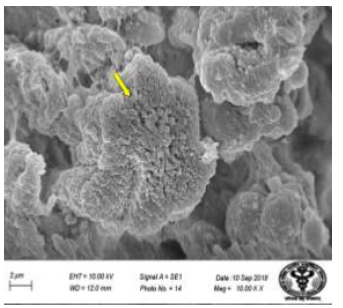
Figure 26: Scanning electron micrograph of duodenum of control rat showing epithelial cells with microvilli (↑). X 10000.
In the jejunum of control rat, hexagonal arrangement of epithelial cells on the villus surface was observed (Figure 33). The scanning electron micrographic examination revealed the normal intestinal epithelium with regular arrangement of microvilli (Figure 34). In the jejunal mucosa of fluoridated rats, several cells had lost their brush border. There were numerous swollen cells clearly separated from one another (Figure 35). The microvilli showed deformation, disruption and corrugated appearance (Figure 36). Within the core of villus surface an extravasation of erythrocyte was prominent and desquamated epithelium was seen. (Figure 37,38). The leaf-like villi appeared distorted and damaged. At the base of these villi, a network of cells was observed, formed by the close contact and interaction of numerous cells. This cellular network highlighted the structural disruptions and cellular alterations caused by fluoride exposure (Figure 39). The uneven mucosal surface exhibited fenestrations and focal protuberances, indicating localized areas of bulging or swelling (Figure 40).
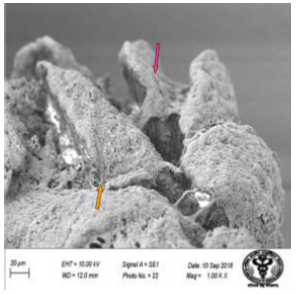
Figure 27: Scanning electron micrograph of duodenum of rat treated with 600 mg sodium fluoride showing erosions (↑) and fissures (↑). X 1000.
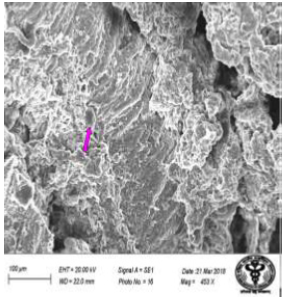
Figure 28: Scanning electron micrograph of duodenum of rat treated with 600 mg sodium fluoride showing extravasion of erythrocyte (↑). X 453.
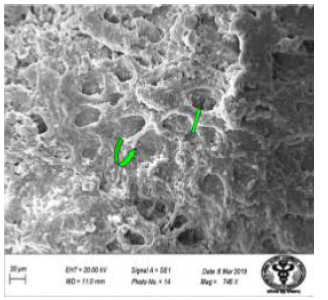
Figure 29: Scanning electron micrograph of duodenum of rat treated with 600 mg/kg b.w /day of NaF for 40 days and showing absence of villi and wide crypt mouths (↑). X 746.
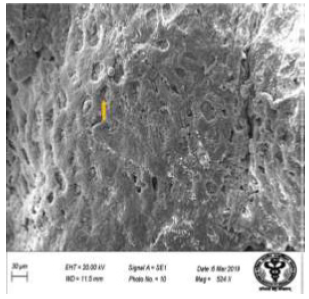
Figure 30: Scanning electron micrograph of duodenum of rat treated with leaf extract of 600 mg/kg b.w./day of NaF for 40 days showing mucosa and corrugations (↑). X 524.

Figure 31: Scanning electron micrograph of duodenum of rat treated with 600 mg of sodium fluoride showing corrugations (↑) and goblet cell orifices (↑). X 2600.

Figure 32: Scanning electron micrograph of duodenum of rat treated with 600 mg of sodium fluoride showing erosions and autolysis of leaflike villi (↑) and a bleb (↑). X 513.
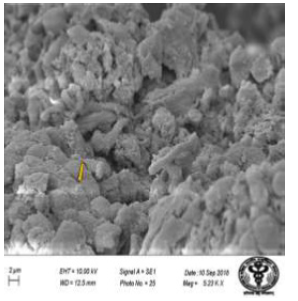
Figure 33: Scanning electron micrograph of jejunum of control rat showing hexagonal arrangement of epithelial cells (↑). X 5230.

Figure 34: Scanning electron micrograph of jejunum of control rat showing normal intestinal epithelium with regular arrangement of microvilli. X 2200.

Figure 35: Scanning electron micrograph of jejunum treated with 600 mg sodium fluoride for 40 days showing swollen cells (↑). X 1650.
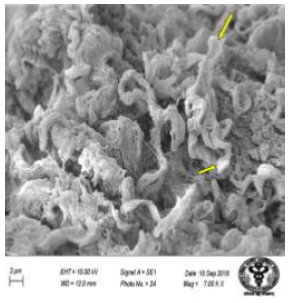
Figure 36: Scanning electron micrograph of jejunum treated with 600 mg sodium fluoride for 40 days showing swollen cells (↑). X 7000.

Figure 37: Scanning electron micrograph of jejunum treated with 600 mg sodium fluoride showing extravasions of red blood cells (↑). X 1390.

Figure 38: Scanning electron micrograph of jejunum of rat treated 600 of NaF for 40 days showing desquamation of epithelial cells (↑) and extravasion of erythrocyte (↑). X 581.

Figure 39: Scanning electron micrograph of jejunum treated with 600 mg sodium fluoride showing distorted leaf-like villi (↑) and cellular network (↑). X 287.

Figure 40: Scanning electron micrograph of jejunum treated with 600 mg sodium fluoride showing uneven mucosal surface with fenestrations and focal protubations. X 2760.
Scanning electron microscopic examination of the ileum from the control rat revealed typical leaf like villi with smooth microvillous surface (Figure 41). A prominent tongue shaped villous tip with a broad base and blunt apex was also observed in the ileum of control rat (Figure 42). In fluoridated rats, fused villi which give doughnut like appearance and Paneth cells were also present (Figure 43). The ileal mucosa of fluorotic rats revealed erosions. At the villous surface, stratified cells and some exfoliated cell sheets were also seen in several regions (Figure 44). The fluorotic ileal mucosa had fissures at the base of villi were present. The tips were curved and broken and showed signs of erosions (Figure 45). SEM examination of ileum from fluoridated rats showed corrugated appearance and the villi abruptly became stubbier, slightly curved and swollen (Figure 46).
Transmission Electron Microscopic Changes
In the duodenum of the control rat, the enterocytes had a regular microvillus border, tiny finger-like projections and a few granular bodies (Figure 47). In the intranuclear region of the enteroendocrine cells, numerous tiny, electron-rich spheroidal granules were present (Figure 48). The microvilli were reduced in height and were damaged (Figure 49). The fluoridated duodenal mucosa had fat droplets suggesting lipid accumulation, vacuolations, a thickened epithelial membrane, multivesicular structures exhibiting endocytic activity and discernible widening of spaces (Figure 50). There were aberrant absorptive cells, granular bodies along mitochondria (Figure 51). The endoplasmic reticulum exhibited vacuolization. Goblet cells were seen among the cellular protrusions (Figure 52). The duodenal mucosa in fluoridated rats appeared flat with the absence of villi, indicating significant damage to the intestinal structure essential for nutrient absorption (Figure 53). Irregular nuclei along with some vacuolation were evident in fluorotic rats. (Figure 54). The jejunum in the control rat revealed normal mitochondria distinctly characterized by its outer membrane and well-developed cristae (Figure 55). Oval basal nuclei surrounded by nuclear membrane were visible in the cells. Several secretory granules and vesicles were present in the cytoplasm (Figure 56). Small intestinal crypts of Lieberkühn included Paneth cells, specialized epithelial cells. The nuclear membrane enclosing the basal nucleus were also seen (Figure 57).
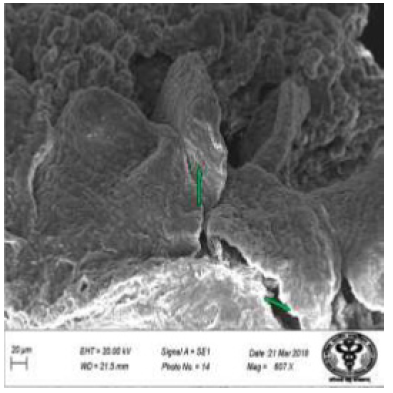
Figure 41: Scanning electron micrograph of ileum of control rat showing leaf like villi with smooth microvillous surface. X 607.
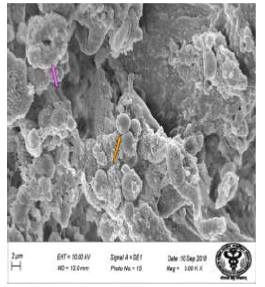
Figure 43: Scanning electron micrograph of ileal tissue treated with 600 mg sodium fluoride for 40 days showing doughnut shaped appearance of villi (↑), caused by their indented tips and presence of paneth cells (↑). X 5000.

Figure 44: Scanning electron micrograph of ileum of rat treated with 600 mg/kg b.w./day of NaF for 40 days showing apparent erosions (↑) stratification and exfoliation of cells (↑). X 731.
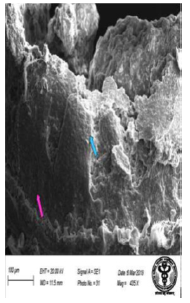
Figure 45: Scanning electron micrograph of ileum of rat treated with 600 mg/kg b.w./day of NaF for 40 days showing fissures (↑) and damaged villi (↑). X 435.
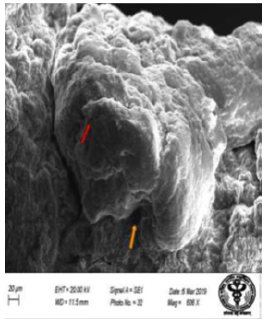
Figure 46: Scanning electron micrograph of ileum of rat treated with 600 mg/kg b.w./day of NaF for 40 days showing corrugations (↑), curved and swollen villi (↑). X 606.
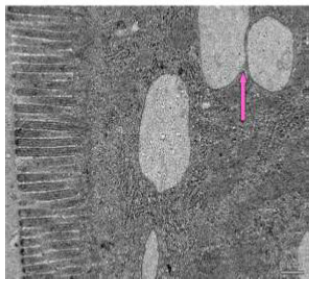
Figure 47: Transmission electron micrograph of duodenum of control rat showing typical appearance of microvilli brush border and granular bodies (↑). X 5000.

Figure 48: Transmission electron micrograph of duodenum of control rat showing enteroendocrine cells with small, spheroidal, electron dense granules (↑) in infranuclear region. X 1100.

Figure 49: Transmission electron micrograph of duodenum of rat treated with 600 mg sodium fluoride for 40 days showing reduced microvilli (↑). X 570.
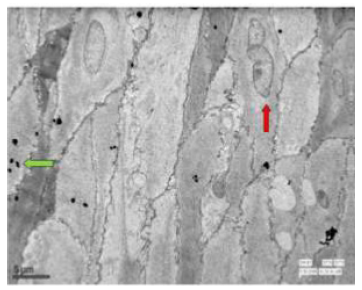
Figure 50: Transmission electron micrograph of duodenum of rat treated with 600 mg sodium fluoride for 40 days showing small fat droplets (↑) and multivesicular bodies showing endocytic activity (↑). Widening of spaces was also observed. X 570.
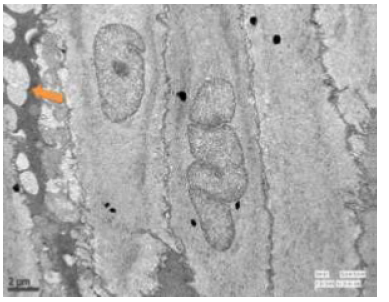
Figure 51: Transmission electron microscopy of duodenum of rat treated with 600 mg sodium fluoride for 40 days showing abnormal granular bodies (↑) along with fragmented mitochondria. X 1100.

Figure 52: Transmission electron micrograph of duodenum of rat treated with 600 mg sodium fluoride for 40 days showing vacuolization of endoplasmic reticulum. Cellular extrusion showing presence of goblet cell was also seen. X 570.

Figure 53: Transmission electron microscopy of duodenum of rat treated with 600 mg sodium fluoride for 40 days showing flattened mucosa and absence of villi. X 7000.
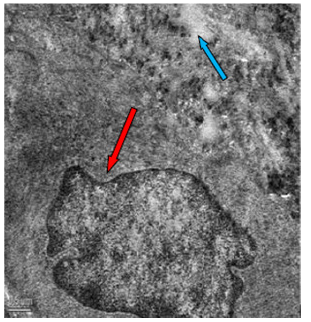
Figure 54: Transmission electron microscopy of duodenum of rat treated with 600 mg sodium fluoride for 40 days showing irregular nuclei (↑) and vacoulations (↑). X 5000.
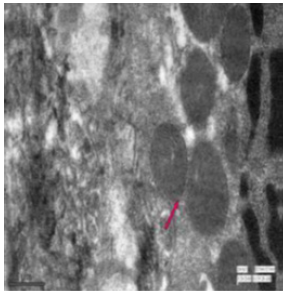
Figure 55: Transmission electron micrograph of jejunum of control rat showing mitochondria (↑) with outer membrane and cristae. X 7000.
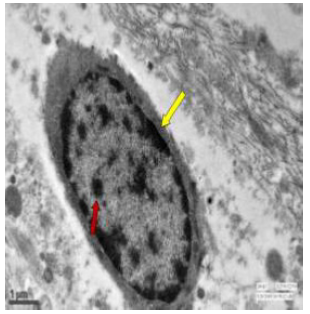
Figure 56: Transmission electron micrograph of jejunum of control rat showing nucleus surrounded by nuclear membrane (↑). The cytoplasm had secretory granules (↑) and vesicles. X 2550.

Figure 57: Transmission electron micrograph of jejunum of rat treated with 600 mg of sodium fluoride showing disruptions in nucleus, mitochondria (↑) and Paneth cells (↑). X 1100.
In the fluoride-treated rat, the endoplasmic reticulum had dilated cisternae, contained the virus particles, characterized by an electron-dense core enveloped by one or two membranes (Figure 58). Under the microvilli, a noticeable terminal web was seen and prominent lateral interdigitations were visible between the cells, suggesting complex folding or invaginations of cell membranes. Additionally, the mitochondria appeared swollen with few disintegrated cisternae (Figure 59). The jejunum of fluorotic rats displayed vacuolations. The presence of lysosomes and vesiculated RER was observed in the cytoplasm (Figure 60).

Figure 58: Transmission electron micrograph of jejunum of rat treated with 600 mg fluoride for 40 days showed virus particles (↑) having electron dense core surrounded by one or two membranes. X 5000.
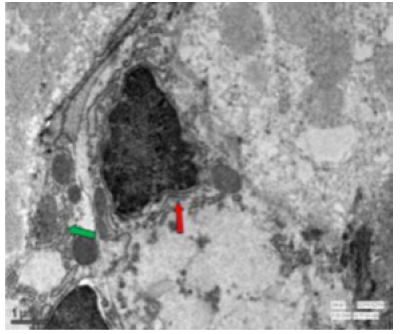
Figure 59: Transmission electron micrograph of jejunum of rat treated with 600 mg fluoride showing interdigitations (↑) swollen mitochondria with few disintegrated cristae. Dilated reR (↑) was also seen. X 2550.
In the ileum of control rat, distinct defensin-rich granules were visible within the Paneth cells, indicating their role in innate immune defence. Furthermore, mitochondria and different secretory granules were also present (Figure 61) and had rough endoplasmic reticulum throughout the cytoplasm (Figure 62). In fluoridated rats, the Golgi apparatus of the ileal tissue appeared aberrant and hypertrophied. The mitochondria had lost their characteristic appearance and were obscured suggesting that high fluoride exposure disrupts cellular structures and functions within the ileum (Figure 63). Significant vacuolations, suggestive of the presence of spaces within the tissue, disintegrated mitochondria and irregularly shaped nuclei were visible (Figure 64). The ileum exhibited irregularities in the rough endoplasmic reticulum (rER) and disruptions in other cytoskeletal components (Figure 65).
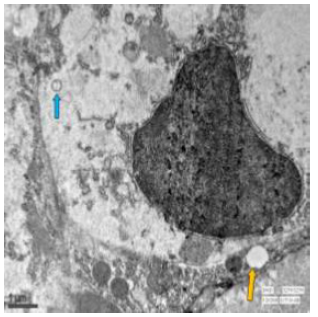
Figure 60: Transmission electron micrograph of jejunum of rat treated with 600 mg sodium fluoride showing vacoulations (↑), vesiculated reR (↑) and some lysosomes. X 2550.
Figure 61: Transmission electron micrograph of ileum of control rat showing rough endoplasmic reticulum. X 2550.
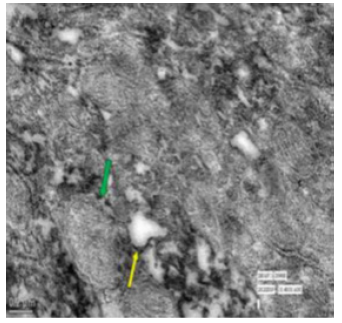
Figure 62: Transmission electron micrograph of ileum of rat treated with 600 mg fluoride showing abnormal mitochondria (↑) and abnormal golgi apparatus (↑). X 9900.

Figure 63: Transmission electron micrograph of ileum of rat treated with 600 mg fluoride showing vacoulations (↑), disintegrated mitochondria. X 7000.
Discussion
Fluoride is a widely recognized environmental pollutant with potential adverse effects on various biological systems. The small intestine, being a critical component of the digestive system, is particularly susceptible to fluoride toxicity. One of the most significant structural alterations observed in the small intestine due to sodium fluoride exposure is villus atrophy. The villi, which are finger-like projections lining the intestinal wall, play a crucial role in nutrient absorption by increasing the surface area. Studies have shown that fluoride exposure leads to a marked shortening and thinning of these villi, significantly reducing their surface area [12]. Histopathological examination revealed that the villi in fluoride-exposed rats were not only shortened but also exhibited a disorganized and fragmented appearance. The villus tips often showed signs of blunting and fusion, further contributing to the reduction in effective absorptive surface area. This morphological change was accompanied by decrease in the number of microvilli on the epithelial cells, exacerbating the absorption issues [13]. Sodium fluoride exposure induces significant degeneration of the intestinal mucosa. This degeneration includes various pathological changes such as epithelial cell shedding, vacuolation, and necrosis [14]. Goblet cells, which secrete mucus, play a vital role in protecting the intestinal epithelium by forming a protective mucus layer. Sodium fluoride exposure leads to a significant reduction in the number of goblet cells, resulting in decreased mucus secretion. The structural alterations, particularly villus atrophy and mucosal degeneration, lead to a significant reduction in the absorptive surface area of the small intestine [15].
The loss of goblet cells and the associated decrease in mucus production compromise the intestinal barrier function [16]. This barrier dysfunction makes the intestine more permeable to pathogens, toxins, and antigens. Sodium fluoride induces an inflammatory response into the mucosa and submucosa. This chronic inflammation can further exacerbate tissue damage and impair intestinal function [17]. The histopathological changes observed in this study are consistent with those reported in other studies. It has been previously shown that fluoride exposure leads to villous atrophy and mucosal inflammation [18]. The use of scanning electron microscopy provides detailed insights into the surface changes occurring at the cellular and subcellular levels in response to NaF exposure. Studies have demonstrated that sodium fluoride induces significant alterations in the structural integrity of the small intestine in rats. These changes can be observed through SEM, highlighting the extent of damage and providing a better understanding of the underlying mechanisms.
SEM analysis has revealed significant disruption of the microvilli on the surface of the intestinal epithelial cells. These fine, hair-like structures, essential for nutrient absorption and enzyme secretion, appeared shortened, fragmented, disorganized and reduced in number in NaF-treated rats. Tier [19] documented mucus discharge from goblet cell orifices. The villi, especially those in the duodenum, have more matured surface epithelial cells with both long and short microvilli. In the small intestine of humans and other animals, three varieties of villi have been identified: finger-shaped, tongue-shaped, and leaf-like villi [20]. Simple tubular glands, crypts of Lieberkühn, were found among the villi [21]. RBC extravasation has been observed in certain villous surface areas. Hexagonal patterns generated by the closely packed apical surfaces of columnar cells become visible [22]. These distinctive hexagonal patterns are indicative of the orderly arrangement and integrity of the epithelial cells under normal conditions. However, exposure to sodium fluoride can disrupt these patterns, further emphasizing the cytotoxic effects on the epithelial lining of the small intestine.
TEM studies have revealed significant ultrastructural alterations in the small intestine of rats exposed to sodium fluoride. Degeneration of cell organelles, including the endoplasmic reticulum, Golgi body, mitochondria, nucleus, mucous membrane, and villi, and severe damage to the irregularly shaped fenestrated cell organelles were among the morphological consequences of the fluoride administration, as revealed by the ultrastructural analysis. TEM images showed swelling of mitochondria, a disruption of the endoplasmic reticulum, and an increase in electron-dense granules within these cells. These changes suggested that fluoride interferes with cellular metabolism and protein synthesis. The present study demonstrated mitochondrial injury and rough endoplasmic reticulum proliferation in fluoride-treated rats [23,24]. In the tissues, black particles emerged. Several ancillary approaches involving light microscopy [25] and these black granules were lipid droplets [26]. In present study, the mucosa appeared flat, and the villi were practically non-existent. Loss of brush border, irregular nuclei and vacuolation of some enterocytes were observed using transmission electron microscopy. The villi exhibited shortening, blunting and distortion [27]. Intestinal defensin expression in Paneth cells was employed as a developmental marker for Paneth cell maturation [28].
Conclusion
The study concluded that sodium fluoride causes significant changes in the ultrastructure of small intestine, as observed through light and electron microscopy. These detailed morphological assessments offer an anatomical foundation for further pathology research on the intestinal segment and demonstrate the effectiveness of using multiple histological methods.
Animal Welfare Statement
The experimental protocols were performed under the approval of Institutional Animal Ethical Committee of Punjabi University, Patiala (Animal maintenance and Registration No. 107/GO/ReBi/S/99/CPCSEA/2017-41).
Conflict of Interest Statement
None.
Acknowledgement
The authors are thankful to all faculties and staff associated with SAIF facility at All India Institute of Medical Sciences, New Delhi, India.
References
- Shashi A, Meenakshi G (2015) Inhibitory Effect of Fluoride on Na+,K+ ATPase Activity in Human Erythrocyte Membrane. Biol Trace Elem Res 168(2): 340-348.
- Shashi A, Kumar M, Bhardwaj M (2008) Incidence of skeletal deformities in endemic fluorosis. Tropical Doctor 38(4): 231-233.
- Shashi A, Bhardwaj M (2011) Prevalence of dental fluorosis in endemic fluoride areas of Punjab, India. Bioscience Biotechnology Research Communications 4(2): 155-163.
- Shashi A, Kumar J (2016) Neurotoxicity induced by fluoride in rat cerebral cortex. International Journal of Current Microbiology and Applied Sciences 5(10): 938-951.
- Shashi A, Dogra S (2024) Biochemical markers of myocardial injury and ultrastructural cardiomyopathy following fluoride administration in rats. World J Pharm. Life Sci 10(8): 113-123.
- Susheela AK, Das TK, Gupta IP, Tandon RK, Kacker SK, et al. (1992) Fluoride ingestion and its correlation with gastrointestinal discomfort. Fluoride 25(1): 5-22.
- Gharzouli K, Senator A (1994) Fluoride absorption in vitro by the gastrointestinal tract of the rat. Fluoride 27(4): 185-188.
- Shashi A (2002) Histopathological effects of sodium fluoride on the duodenum of rabbits. Fluoride 35(1): 28-37.
- Sharma JD, Jain P, Sohu D (2009) Gastric discomforts from fluoride in drinking water in Sanganer Tehsil, Rajasthan, India. Fluoride 42: 286-291.
- Drury AR, Wallington EA (1967) Carleton’s histological techniques. 5th edn. Oxford University Press. London 140.
- Karnovsky MJ (1965) A formaldehyde-glutarldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27: 137-138.
- Shashi A, Thapar SP (2001) Histopathology of small intestine in experimental fluorosis in rabbits. Fluoride 34(1): 36-42.
- Smith JA, Doe RB (2020) Histological impact of fluoride exposure on the intestinal morphology of rats. J Exp Biol 45(3): 123-134.
- Das K, Mukherjee D (2017) Histopathological changes of liver and intestine in sodium fluoride intoxicated rat. Indian J Exp Biol 55(4): 249-256.
- Liu J, Xia T, Zhang M, He W, Wu D, et al. (2014) Oxidative stress in the rat's small intestine induced by different concentrations of sodium fluoride in drinking water. Chemosphere 112: 1-6.
- Wang J, Ge Y, Ning H, Wang S (2012) Protective effects of selenium against apoptosis in rat intestine induced by fluoride. Biol Trace Elem Res 145(1): 150-157.
- Varol E, Icli A, Aksoy F, Basar N, Sutcu R, et al. (2013) Evaluation of total oxidative status and oxidative DNA damage in patients with endemic fluorosis. Food Chem.Toxicol 59: 324-328.
- Christine B, Moore, Richard L Young (2017) Comparative Histopathological Analysis of Fluoride Effects on Gastrointestinal Tract. Comp Biochem Physiol 215(1): 62-70.
- Tier RA (1963) Scanning electron microscopy of the intestinal mucosa in various experimental conditions. J Microsc 82(2): 123-135.
- De Ocampo, DM Sison JR (1983) Scanning electron microscopic study of the small intestine in fluoride-exposed rats. Fluoride 16(3): 143-152.
- Leslie JA, James MP (2007) Detailed scanning electron microscopy of intestinal damage in experimental models of gastrointestinal disorders. Int J Gastrointest Pathol 12(1): 75-84.
- Toner PG, Carr KE (1969) Electron microscopy of normal and abnormal intestinal villi. Gut 10(9): 678-687.
- Elbetieha A, Al Khalili R, Taha R (2000) The effect of fluoride on the rat small intestine: a transmission electron microscope study. J Fluor Chem 103(1): 57-64.
- Xu B, Liu X Liu Y (2008) Fluoride-induced changes in the rat small intestine as observed by transmission electron microscopy. Fluoride 41(3): 195-200.
- Sjölund K, Sandén G, Håkanson R, Sundler F (1983) Endocrine cells in human intestine: An immunocytochemical study. Gastroenterol 85(5): 1120-1130.
- Blanshard C, Ellis DS, Tovey G, Gazzard BG (1993) Electron microscopy of rectal biopsies in HIV-positive individuals. J Pathol 169: 79-87.
- Atiq A, Shal B, Naveed M, Khan A, Ali J, et al. (2019) Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur J Pharmacol 15(843): 292-306.
- Mallow EB, Harris A, Salzman N, De Berardinis RJ, Ruchelli E, et al. (1996) Human enteric defensins: Gene structure and developmental expression. J Biol Chem 271(8): 4038-4045.





 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.